Learning Outcomes
After completing this module, you should be able to:
- Predict how ions will move across a membrane according to their permeability and the electrochemical gradient (concentration gradient plus voltage gradient).
- Calculate the equilibrium membrane potential for each ion using the Nernst equation.
- Use the Goldman equation to predict the membrane potential during an action potential.
Getting through membranes
Water is a small, polar molecule that can get through the cell membrane pretty easily due to the presence of water channels (aquaporins) which are always open and act like a tube connecting one side of the membrane to the other.
Other larger or charged molecules find it harder to cross the cell membrane, but they are not completely blocked.
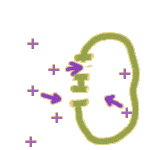 In fact, there are two major ways that an ion like Na+ can make it through the membrane:
In fact, there are two major ways that an ion like Na+ can make it through the membrane:
1. Through a channel: Membrane channels can open and close like gates. Once a channel is open, ions are free to move in both directions -- but the NET movement of ions will be down a gradient. (More on that later.)
2. 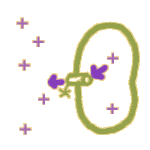 By a pump: Membranes also contain tiny ATP-driven protein pumps. When these pumps operate, the energy stored in ATP is used to move ions in ONE direction only -- whichever direction the pump mechanism works. This allows ions to be pushed around AGAINST a gradient.
By a pump: Membranes also contain tiny ATP-driven protein pumps. When these pumps operate, the energy stored in ATP is used to move ions in ONE direction only -- whichever direction the pump mechanism works. This allows ions to be pushed around AGAINST a gradient.
Copyright University of Maryland, 2007
You may link to this site for educational purposes.
Please do not copy without permission
requests/questions/feedback email: mathbench@umd.edu